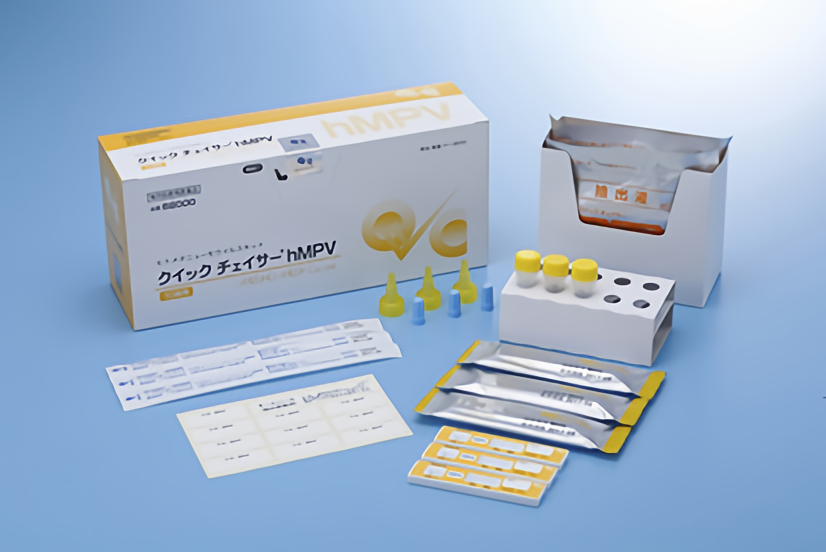
Instructions For Use
[General precautions]
1) For in vitro diagnostic use only
2) The diagnosis of human metapneumovirus infection should be
comprehensively made not only by test result of this product,
but also in conjunction with the assessment of clinical progress
and results of other tests.
3) Procedures which are not described in instruction for use, are
not guaranteed.
[Contents]
1) Test plate- 10 tests
・Mouse monoclonal anti- human metapneumovirus antibodies
・Colloidal gold conjugated to mouse monoclonal anti-human metapneumovirus antibodies
2) Extraction reagent solution vial – 10 vials
Extraction reagent solution is buffer containing detergent.
3) Swab (for nasopharyngeal swab specimen & for nasal aspirate specimen) – 10 pieces
4) Rack (for extraction reagent solution vial) – 1 piece
5) Filter (for extraction reagent solution vial) – 10 pieces
6) Blue cap (for temporary storage of extraction reagent solution vial) – 10 pieces
7) Name label for extraction reagent solution vial – 1 sheet
[Intended Use]
For detection of human metapneumovirus in nasopharyngeal swab specimen or nasal aspirate specimen
(Aid in the diagnosis of infection by human metapneumovirus)
[Principle of the test]
「Quick Chaser ® hMPV」 is the in-vitro reagent for detection of human metapneumovirus antigen based on Immunochromatographic Assay.
Colloidal gold conjugated to mouse monoclonal anti-human metapneumovirus antibodies and colloidal gold conjugated to rabbit immunoglobulin for control line are coated in sensitized colloidal gold coating area in test plate. And, mouse monoclonal anti- human metapneumovirus antibodies are immobilized in test line area and anti-rabbit immunoglobulin antibodies for control line are immobilized in control line area.
According to immunochromatographic principle, when samples are added to the sample area, in the presence of human metapneumovirus, they migrate to the area between sample area and test line area, where reacting with colloidal gold conjugated to mouse monoclonal anti-human metapneumovirus antibodies, and
react with mouse monoclonal anti-human metapneumovirus antibodies and they are caught in test line area.
As the result, purple- red line is visible. Moreover, purple-red line is also simultaneously visible for catching colloidal gold conjugated to rabbit immunoglobulin by anti-rabbit immunoglobulin antibodies in control line area, regardless of presence of human metapneumovirus.
[Procedural precautions]
1) Do not use saliva and sputum as a specimen.
2) Collected specimen should be prepared as sample in accordance with after-mentioned “Preparation of sample in Test procedure” and tested as soon as possible.
3) Add fixed volume (3 drops) to the center of sample area from tip of filter about 10mm away from the sample area so as to make droplets. In case of adding other than fixed volume, an accurate reaction may not be performed.
4) Bring test plate and extraction reagent solution to 15 ~30 ℃ prior to testing.
5) Keep interpretation time inevitably because it causes false-negative and false-positive.
6) Interfering substances and medications
The following substances and blood did not interfere the performance of this product at the concentration listed below:
Acetyl salicylate (10 mg/ml)
Ibuprofen (20 mg/ml)
Diphenhydramine hydrochloride (5 mg/ml)
Oxymetazoline hydrochloride (10 mg/ml)
Dextromethorphan hydrogen bromide (5 mg/ml)
Phenylphrine hydrochloride (50 mg/ml)
Cold medicine (concentration of Acetaminophen: 10 mg/ml)
Nasal drop ① , containing Sodium cromoglicate, Chlorpheniramine monomaleate, Naphozoline hydrochloride (20%)
Nasal drop ②, containing Ketotifen fumarate (10%)
Inhalation ① , containing Salbutamol sulphate (20%)
Inhalation ② , containing Bromhexine hydrochloride (20%)
Gargle ① , containing Tincture of Myrrh (0.5 %)
Gargle ② , containing Povidoneiodine (2.0 %)
Intraoral antiphlogistics , containing Sodium Azulene Sulfonate (10 %)
Throat candy ① , containing Di-potassium Glycyrrhizinate (20 mg/ml)
Throat candy ② , containing Nandina Fruit Extract (Dry) (10 mg/ml)
Throat candy ③ , containing Cetylpyridinium chloride (20 mg/ml) Blood (1%)
7) Cross reactivity
Cross reactivity were not observed with the following viruses and bacteria.
・Virus
Influenzavirus A, Influenzavirus B, Adenovirus type 1,
Adenovirus type 2, Adenovirus type 3, Adenovirus type 4,
Adenovirus type 5, Adenovirus type 6, Adenovirus type 7,
Adenovirus type 11, Human Coronavirus, Coxsackie virus A9,
Coxsackie virus B5, Human Echovirus 9, Herpes simplex virus
type 1, Mumps virus, Parainfluenza virus 1, Rhinovirus 8
・Bacteria
Bordetella pertussis, Candida albicans, Haemophilus in!uenzae, Klebsiella
pneumoniae, Listeria monocytogenes, Moraxella catarrhalis, Mycoplasma
pneumoniae, Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus
aureus, Staphylococcus epidermidis, Streptococcus agalactiae(Group B),
Streptococcus mutans, Streptococcus pneumoniae, Streptococcus pyogenes
(Group A)
[Test procedure]
● Specimen collection
1) Preparation of specimen collection
① Swab : Use swab, included in this test kit.
② Extraction reagent solution: Use it without preparation.
2) Specimen collection
① Nasopharyngeal swab specimen:
 Along inferior nasal conchae (imaginga horizontal plane connecting nostril with external acoustic meatus ),insert swab in nasal cavity and rub it on the mucosal surface several times to collect
Along inferior nasal conchae (imaginga horizontal plane connecting nostril with external acoustic meatus ),insert swab in nasal cavity and rub it on the mucosal surface several times to collect
mucous epidermis.
Note) Elastic plastic rod is employed in swab to reduce the burden of patients. However, on the other hand, the tip of swab may not be reached to inflamed region on the wall of nasal cavity or you may not be able to rub the tip on the wall of nasal cavity adequately to collect enough volume of virus antigen, even though the tip of swab is reached to inflamed region on the wall of nasal cavity. Therefore, make sure the tip of swab is reached to the wall of nasal cavity so as to rub the inflamed region at the time of collecting mucous epidermis.
② Nasal aspirate specimen:
Dip spherical tip into low-viscosity liquid part of nasal aspirate specimen in trap. If it is difficult to collect specimen due to high viscosity or low volume of specimen, add 0.5~1mL of saline to specimen, and mix them uniformly. Test can be performed by using the specimen.Be reminded that sensitivity is decreased by dilution of specimen with saline.
● Details of Extraction reagent solution vial
● Preparation of sample
● Details of test plate
● Test procedure
1) Preparation of reagent
Test plate: No prior preparation required
2) Test procedure
① Take out test plate from aluminum foil pouch. Remove
desiccant sheet.
② Avoiding contact of tip of extraction filter with sample area,
add 3 drops (About 110 μL) of sample to sample area of test
plate from extraction reagent solution vial including prepared
sample.
③ Leave to react at 15~30℃. Visually interpret test result by
lines in test line area and control line area after 5 ~10
minutes.
[Interpretation]
Interpret by existence of red-purple lines in test line area and
control line area.
《Positive》Test line and control line appear.

Positive
《Negative》Only control line appears.
Negative
《Retest》
If both test line and control line do not appear or no control line appear, operational mistakes such as the lack of sample are thought. Recheck test procedure and retest with new test plate. If the same result comes out in the retest again, confirm it with other method.
Retest
Retest
● Interpretational precautions
1) In case test line and control line appear at 5~10 minutes after dropping sample, it can be interpreted as positive. Negative should be interpreted at 10 minutes after dropping sample.
Streak line might appear before 10 minutes temporarily. Do not interpret the temporal streak line as appearance of test line. After 10 minutes, colloidal gold can appear like line due to drying of test plate with time. Therefore, please interpret test results at 10 minutes.
2) This product is used as aid in the diagnosis for infection of human metapneumovirus. In case human metapneumovirus in specimen are below the detection limit of the test or specimen collection are not enough, test result could be interpreted as negative, even though patients are infected by human metapneumovirus. Moreover, factors in specimen could cause non-specific reaction and negative specimen could be interpreted as positive. The definitive diagnosis should be made comprehensively in conjunction with the assessment of clinical progress and results of other tests for confirmation.
[Performance characteristics]
1) Performance
① Sensitivity
・When in house human metapneumovirus positive control Note
1) was tested, human metapneumovirus positive result was obtained.
② Accuracy
・When in-house human metapneumovirus positive control was tested, human metapneumovirus positive result was obtained.
・When in-house negative control Note 2) was tested, negative result was obtained.
③ Reproducibility
・When in-house human metapneumovirus positive control were tested three times simultaneously, human metapneumovirus positive results were obtained in all cases.
・When in-house negative controls were tested three times simultaneously, negative results were obtained in all cases.
Note 1) a human metapneumovirus purified antigen are diluted by extraction reagent solution to become the 9.57×10 6 copies/mL.
Note 2) Extraction reagent solution
④ Detection limit
Human metapneumovirus Subtype A1 2.39×106copies/mL
Human metapneumovirus Subtype B1 2.99×105copies/mL
⑤ Reactivity
It is confirmed that this product reacts with human metapneumovirus A1, A2, B1 and B2.
2) Correlations
Comparison with existing products
● Nasopharyngeal swab specimen
Positive agreement rate : 100%(51/51)
Negative agreement rate:95.3%(61/64)
Total agreement rate :97.4%(112/115)
※1 Negative by other product (1), but positive by Quick Chaser RSV/hMPV.
The three samples were positive by RT-PCR.
…………………………………………………………………………………………………………………
Positive agreement rate :100%(51/51)
Negative agreement rate:95.3%(61/64)
Total agreement rate :97.4%(112/115)
※2 Negative by other product (2), but positive by Quick Chaser RSV/hMPV. The three samples were positive by RT-PCR.
● Nasal aspirate specimen
Positive agreement rate : 100%(52/52)
Negative agreement rate:96.3%(52/54)
Total agreement rate :98.1%(104/106)
※3 Negative by other product (1), but positive by Quick Chaser RSV/hMPV. The two samples were positive by RT-PCR.
3) Calibration reference material (Standard material)
Human metapneumovirus antigen solution (in-house standard)
[Precautions for use, handling]
1) Precaution for handling (Prevention of danger)
① HIV, HBV, and HCV could be included in specimen. Be careful of handling specimen as potentially infectious material.
② Be careful not to touch sample (specimen) or extraction reagent solution directly to skin or not to get them into eyes, wearing glasses, disposable glove or mask at the time of use.
③ Do not use swab to collect specimen, if it is already put into extraction reagent solution.
④ If specimen (sample) or extraction reagent solution are got into eyes or mouth, flush with a plenty of water as emergency treatment and see a doctor, if necessary.
⑤ Do not use blue cap which belong to kit for transporation or preservation because it does not have seal stregnth.
⑥ Perform the collection of specimen under the guidance of the qualified person.
⑦ Raw material of membrane which is used for test plate, is nitrocellulose. Do not perform test near fire because nitrocellulose is extremely flammable material.
⑧ Regarding aspiration tube with trap for nasal aspirate specimen, use unused and contamination-free one per test not to cause contamination for preventing infection spread and maintaining accuracy of test.
⑨ Wipe off with alcohol for disinfection in case of getting splattered with sample (specimen).
2) Precaution for use
① Do not freeze this product. Store it in accordance with description of instructions for use. Do not use frozen reagents because they could show false results by change of quality.
② Do not use this product beyond expiration date.
③ Do not store extraction reagent solution vial with laying down or inverting.
④ Use extraction reagent solution included in this kit. Do not use extraction reagent solutions of other products.
⑤ Immediately use test plate after opening of foil pouch. If it is left for long time after the opening, it could be non-reactive due to getting moistened.
⑥ Do not touch sample area, test line area and control line area by hands directly.
⑦ Do not perform test in the place such as under air conditioner where the dry wind directly blows the surface of test plate, to prevent uneven migration.
⑧ Do not use reagent, accessaries etc. of the product except the purpose of this product.
⑨ Test plate, swab, and extraction reagent solution vial (Filter, yellow cap and blue cap are included) are intended for single use only.
⑩ Use swab, included in this product.
⑪ Store swab avoiding direct sunlight, high temperature and humidity with being careful about the water wets.
⑫ Do not touch spherical tip of swab before use.
⑬ When swab is taken out of a pouch, do not press spherical tip (sponge) and rod (handle) of the swab over the pouch by hand fingers. Load on the spherical tip may make spherical tip secede or drop spherical tip off from the rod.
⑭ Use swab immediately after opening the pouch.
⑮ If break and/or hole are found on the pouch of swab, do not use it.
⑯ If swab is stained, broken or bent, do not use it.
⑰ Do not bend and curve the rod of swab before collecting specimen.
⑱ Be careful not to break the rod of swab and injure region to be collected (mucous epidermis) by excessive force or pushing too hard at the time of specimen collection.
⑲ Elastic plastic rod is employed in swab to reduce the burden of patients. However, on the other hand, the tip of swab may not be reached to inflamed region on the wall of nasal cavity or you may not be able to rub the tip on the wall of nasal cavity adequately to collect enough volume of virus antigen, even though the tip of swab is reached to inflamed region on the wall of nasal cavity. Therefore, make sure the tip of swab is reached to the wall of nasal cavity so as to rub the inflamed region at the time of collecting mucous epidermis.
⑳ Be careful not to splatter the sample at the time of taking the swab out of vial after preparing sample. In case collection volume of specimen is excess, or
high-viscosity, membrane filter could be clogged and adequate sample volume could not be dropped. In such cases, collect specimen again and retest it with new test plate.
3) Precaution for waste disposal
① Treat liquid waste and used utensils by any one of following methods because sample (specimen) could contain infectious material such as HIV, HBV, and HCV etc.
a) Immerse in sodium hypochlorite solution (effective chlorine concentration of 1000ppm) for more than 1 hour.
b) Immerse in 2% glutaraldehyde solution for more than 1 hour.
c) Autoclave at 121°C for more than 20 minutes.
② Regarding disposal of reagents and utensils etc. , dispose of them as medical waste, industrial waste or infectious waste in accordance with your waste disposal laws and regulations.
[Storage・Expiry]
・Storage: 1~30℃
・Expiry:24 months (As indicated on package)