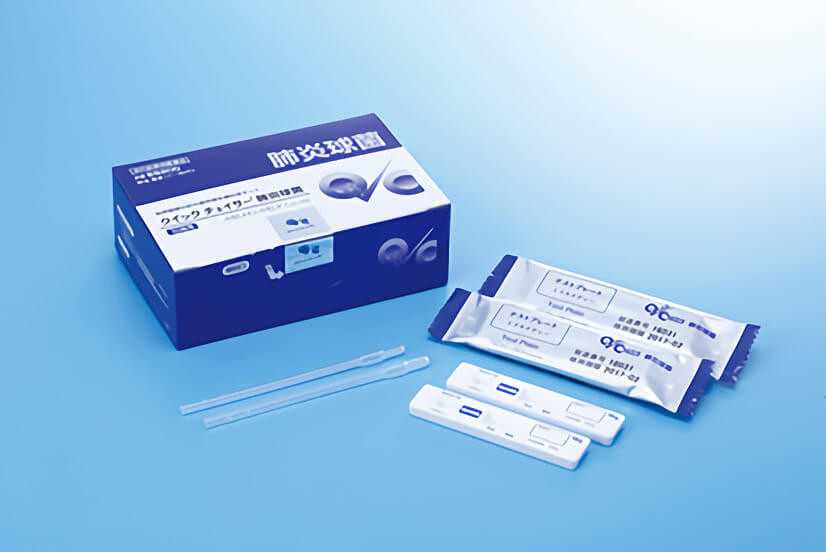
產品特色
♥僅需滴尿即可通過簡單程序輕鬆解釋。
♥5分鐘後即可得出正面結果。 快速解釋是可能的。
♥檢測限:2.5 x 102CFU / mL(高靈敏度)。
Instructions For Use
[General caution]
1) Do not use this product for other purpose than in vitro diagnosis.
2)The diagnosis of streptococcus pneumoniae infection should be
comprehensively made not only by test result of this product, but also in conjunction with the assessment of clinical progress and results of other tests.
3) Other usages than Instructions For Use are not guaranteed.
[Contents]
1) Test plate – 10 tests
・Rabbit polyclonal anti-streptococcus pneumoniae antibodies
・Colloidal gold conjugated to rabbit polyclonal anti-streptococcus pneumoniae antibodies
2) Dropper – 10 pieces
[Intended use]
For detection of streptococcus pneumoniae antigen in urine (An aid of diagnosis for an infection of streptococcus pneumoniae)
[Principle of the test]
Quick Chaser ® Streptococcus Pneumoniae is the in-vitro reagent for detection of streptococcus pneumoniae antigen based on Immunochromatographic Assay.
Colloidal gold conjugated to rabbit polyclonal anti-streptococcus pneumoniae antibodies and colloidal gold conjugated to mouse immunoglobulin for control line are coated in sensitized colloidal gold coating area in test plate. And rabbit polyclonal anti-streptococcus pneumoniae antibodies are immobilized in test line area. Anti-mouse immunoglobulin antibodies for control line are immobilized in control line area.
According to immunochromatographic principle, when samples are added to the sample area, in the presence of streptococcus pneumoniae antigens, they migrate to the area between sample area and test line area, where reacting with colloidal gold conjugated to rabbit polyclonal anti-streptococcus pneumoniae antibodies and moreover, react with rabbit polyclonal anti-streptococcus
pneumoniae antibodies and they are caught in test line area. As a result, purple-red line appears in test line area.
Purple-red line also simultaneously appears for catching colloidal gold conjugated to mouse immunoglobulin by anti-mouse immunoglobulin antibodies in control line area, regardless of presence of streptococcus pneumoniae.
[Procedural notes]
1) Do not use spinal fluid, serum, sputum or pharyngeal swab etc. as a specimen except urine.
2) Do not use turbid urine containing pus or blood etc.
3) Collect specimen with an aseptic container.
4) Collected specimen should be tested as soon as possible. In case that test is not performed immediately or specimen need to be stored for a long time, test specimen within 3 days for storage at 5 ~ 30℃ and within 14 days for storage at -80℃~ 4℃. Do not repeat freezing and thawing of specimen three times or
more. Specimen should be brought to 15 ~ 30℃ prior to use.
5) When using dropper included in kit, take tip of swab out from urine after absorbing urine completely. In case that a large volume of air enter into tip of swab, absorb urine again because urine volume could be insufficient.
6) Add fixed volume (100 μl) of specimen to the center of sample area. As for the case except fixed volume, an accurate reaction may not be conducted.
7) Bring test plate to 15 ~ 30℃ prior to testing.
8) Interfering substances and medications
The following substances and blood did not interfere the performance of this product at the concentration listed below:
Glucose up to 5000 mg/dl
Albumin up to 2000 mg/dl
Blood up to 5%
9) Cross reactivity
Cross reactivity with the following virus and bacteria were not observed.
・Bacteria
Candida albicans, Chlamydophila (Chlamydia) pneumoniae, Citrobacter
freundii, Enterobacter aerogenes, Enterobacter cloacae, Enterococcus faecalis,
Escherichia coli, Haemophilus in!uenzae b, Klebsiella pneumoniae,
Legionella pneumophila, Moraxella catarrhalis, Mycoplasma pneumoniae,
Neisseria gonorrhoeae, Proteus mirabilis, Pseudomonas aeruginosa, Serratia
marcescens, Staphylococcus aureus, Staphylococcus epidermidis,
Streptococcus agalactiae, Streptococcus anginosus, Streptococcus Group A,
Streptococcus Group C, Streptococcus Group F, Streptococcus Group G,
Streptococcus mutans
・Virus
Adenovirus 11, Adenovirus 19, Adenovirus 37,
Influenza virus A , Influenza virus B, Parainfluenza virus 1,
Rhinovirus 8, Respiratory syncytial virus A,
Respiratory syncytial virus B
10) Influence of vaccine
When Streptococcus pneumoniae vaccine is inoculated, an antigen derived from vaccine is exhausted in urine for a few days and be careful because positive test result may be given.
[Test procedure]
● Details of test plate
 ●Test procedure
●Test procedure
1) Preparation of reagent
No prior preparation required.
2) Test procedure
① Take out test plate from aluminum foil pouch.
Remove desiccant sheet included in aluminum foil pouch.
② Absorb urine so that urine can reach between two graduation lines near tip of dropper included in kit. (About 100μL can be collected.) When taking dropper out from urine, get dropper upright and take it out.
③ Add all absorbed urine to specimen area of test plate vertically.
※Add urine of 100μL to test plate in case of using micropipette.
④ Leave to react at 15 ~30℃.
Visually interpret test result by lines appearing in test line area and in control line area after 5 ~10 minutes.
[Interpretation]
Interpret by existence of red-purple lines appearing in test line area and control line area.
《Positive》
Both test line and control line appear.
Positive
《Negative》
Only control line appears.
Negative
《Retest》
If both test lines and control line do not appear or no control line appear, operational mistakes such as the shortage of sample volume etc. are thought about. Recheck test procedure and retest with new test plate. If the same result comes out in the retest again, confirm it with other method.
Retest
Retest
● Interpretational note
1) In case test line and control line appear at 5 ~ 10 minutes after adding sample, it can be interpreted as positive. Negative should be interpreted at 10 minutes after adding sample. Streak line might appear before 10 minutes temporarily. Do not interpret the temporal streak line as appearance of test line. After 10 minutes, colloidal gold can appear like line due to drying of test plate with time. Therefore interpret test results at 10 minutes.
2) This product is used as an aid in the diagnosis for infection of streptococcus pneumoniae. In case that streptococcus pneumoniae antigens in specimen are below the detection limit of the test, test result could be interpreted as negative,
even though patients are infected by streptococcus pneumoniae. Moreover, special factors in specimen could cause non-specific reaction and negative specimen could be interpreted as positive. The definitive diagnosis should be made comprehensively in conjunction with the assessment of clinical progress and other test result.
3) Streptococcus mitis has a common antigen with streptococcus pneumoniae and it is supposed that positive result is given when there is much quantity of the antigen.
4) When streptococcus pneumoniae habitually resides to the nasopharynx in infant, a streptococcus pneumoniae antigen is exhausted in urine and be careful because positive result may be given.
5) It is usually said that quantity of urinary streptococcus pneumoniae antigen reaches detectable sensitivity or more after 3 days after a pneumoniae symptom appeared, but it is different by a symptom. In addition, an antigen may be exhausted in urine from a few days to several weeks after infection.
[Performance Characteristics]
1) Performance
① Sensitivity
・When in-house positive control Note 1) was tested, a positive result was shown.
② Accuracy
・When in-house positive control was tested, a positive result was shown.
・When in-house negative control Note 2) was tested, negative result was shown.
③ Reproducibility
・When in-house positive controls were tested three time simultaneously, positive results were shown in all cases.
・When in-house negative controls were tested three times simultaneously, negative results were shown in all cases.
Note 1) Streptococcus pneumoniae antigen control solution, diluted by artificial urine to be equivalent to 1.0×10 3 CFU/ml of reference material.
Note 2) Aqueous solution including urea and sodium chloride
④ Detection Limit
2.5×10 2 CFU/mL
⑤ Serotype and reactivity
It is confirmed that this product reacts with streptococcus pneumoniae serotype 1, 3, 4, 6B, 9N, 9V, 10A, 11A/E, 12F, 14, 15B, 18C, 19A, 19F, 20, 22F, 23F, 33F.
2) Correlation
Comparison with existing approval product
(immmunochromatographic assay)
Quick Chaser Streptococcus Pneumoniae
Other product
Positive agreement rate : 100%(62/62)
Negative agreement rate :92.7%(51/55)
Total agreement rate :96.6%(113/117)
3) Calibration reference material (Standard material)
Streptococcus pneumoniae (ATCC49619)
[Precaution for use or handling]
1) Precaution for handling (hazard prevention)
①Other infectious materials could be included besides streptococcus pneumoniae in specimen. Be careful of handling specimen as potentially infectious materials.
②Be careful not to touch specimen directly to skin or not to get into eyes in wearing glasses, disposable gloves or mask at the time of use.
③If specimen is got into eyes or mouth, flush with a plenty of water as emergency treatment and see a doctor if necessary.
④Raw material of membrane which is used for test plate, is nitrocellulose. Do not perform test near fire because nitrocellulose is extremely flammable material.
2) Precaution for use
①Avoid freezing of reagent and store it in accordance with description of instruction for use. Do not use frozen reagents because they could show false results by change of quality.
②Do not use this product beyond expiration date.
③Use the test plate immediately after opening aluminum foil pouch. If test plate is left for a long time, it could not react by exposure to moisture.
④Do not touch sample area, test line area and control line area by hands directly.
⑤Do not perform test in the place such as under air conditioner where the dry wind directly blows the surface of the test plate, to prevent uneven migration.
⑥Do not use the reagent and the accessories etc. of this product except the purpose of this testing.
⑦Test plate and dropper are intended for single use only.
3) Precaution for waste disposal
①Treat liquid waste and used utensils by any one of following methods because specimen could contain other infectious material besides streptococcus pneumoniae.
a) Immerse in sodium hypochlorite solution (effective chlorine concentration of 1,000 ppm) for 1 hour or more.
b) Immerse in 2 % glutaraldehyde solution for 1 hour or more.
c) Autoclave at 121℃ for 20 minutes or more.
②Regarding disposal of used reagent and utensils, dispose of them as medical waste, industrial waste or infectious waste in accordance with Local Regulation and Law of waste disposal.
[Storage・Expiry]
・Storage: Room temperature (1~30℃)
・Expiry: 12 months (As indicated on package)
 ♥ Easy interpretation by easy procedure of only dropping urine.
♥ Easy interpretation by easy procedure of only dropping urine.
♥ Positive result can be interpreted from 5 minutes. Speedy interpretation is possible.
♥ Detection limit : 2.5 x 102CFU/mL (High sensitivity).
Instructions For Use
[General caution]
1) Do not use this product for other purpose than in vitro diagnosis.
2)The diagnosis of streptococcus pneumoniae infection should be
comprehensively made not only by test result of this product, but also in conjunction with the assessment of clinical progress and results of other tests.
3) Other usages than Instructions For Use are not guaranteed.
[Contents]
1) Test plate – 10 tests
・Rabbit polyclonal anti-streptococcus pneumoniae antibodies
・Colloidal gold conjugated to rabbit polyclonal anti-streptococcus pneumoniae antibodies
2) Dropper – 10 pieces
[Intended use]
For detection of streptococcus pneumoniae antigen in urine (An aid of diagnosis for an infection of streptococcus pneumoniae)
[Principle of the test]
Quick Chaser ® Streptococcus Pneumoniae is the in-vitro reagent for detection of streptococcus pneumoniae antigen based on Immunochromatographic Assay.
Colloidal gold conjugated to rabbit polyclonal anti-streptococcus pneumoniae antibodies and colloidal gold conjugated to mouse immunoglobulin for control line are coated in sensitized colloidal gold coating area in test plate. And rabbit polyclonal anti-streptococcus pneumoniae antibodies are immobilized in test line area. Anti-mouse immunoglobulin antibodies for control line are immobilized in control line area.
According to immunochromatographic principle, when samples are added to the sample area, in the presence of streptococcus pneumoniae antigens, they migrate to the area between sample area and test line area, where reacting with colloidal gold conjugated to rabbit polyclonal anti-streptococcus pneumoniae antibodies and moreover, react with rabbit polyclonal anti-streptococcus
pneumoniae antibodies and they are caught in test line area. As a result, purple-red line appears in test line area.
Purple-red line also simultaneously appears for catching colloidal gold conjugated to mouse immunoglobulin by anti-mouse immunoglobulin antibodies in control line area, regardless of presence of streptococcus pneumoniae.
[Procedural notes]
1) Do not use spinal fluid, serum, sputum or pharyngeal swab etc. as a specimen except urine.
2) Do not use turbid urine containing pus or blood etc.
3) Collect specimen with an aseptic container.
4) Collected specimen should be tested as soon as possible. In case that test is not performed immediately or specimen need to be stored for a long time, test specimen within 3 days for storage at 5 ~ 30℃ and within 14 days for storage at -80℃~ 4℃. Do not repeat freezing and thawing of specimen three times or
more. Specimen should be brought to 15 ~ 30℃ prior to use.
5) When using dropper included in kit, take tip of swab out from urine after absorbing urine completely. In case that a large volume of air enter into tip of swab, absorb urine again because urine volume could be insufficient.
6) Add fixed volume (100 μl) of specimen to the center of sample area. As for the case except fixed volume, an accurate reaction may not be conducted.
7) Bring test plate to 15 ~ 30℃ prior to testing.
8) Interfering substances and medications
The following substances and blood did not interfere the performance of this product at the concentration listed below:
Glucose up to 5000 mg/dl
Albumin up to 2000 mg/dl
Blood up to 5%
9) Cross reactivity
Cross reactivity with the following virus and bacteria were not observed.
・Bacteria
Candida albicans, Chlamydophila (Chlamydia) pneumoniae, Citrobacter
freundii, Enterobacter aerogenes, Enterobacter cloacae, Enterococcus faecalis,
Escherichia coli, Haemophilus in!uenzae b, Klebsiella pneumoniae,
Legionella pneumophila, Moraxella catarrhalis, Mycoplasma pneumoniae,
Neisseria gonorrhoeae, Proteus mirabilis, Pseudomonas aeruginosa, Serratia
marcescens, Staphylococcus aureus, Staphylococcus epidermidis,
Streptococcus agalactiae, Streptococcus anginosus, Streptococcus Group A,
Streptococcus Group C, Streptococcus Group F, Streptococcus Group G,
Streptococcus mutans
・Virus
Adenovirus 11, Adenovirus 19, Adenovirus 37,
Influenza virus A , Influenza virus B, Parainfluenza virus 1,
Rhinovirus 8, Respiratory syncytial virus A,
Respiratory syncytial virus B
10) Influence of vaccine
When Streptococcus pneumoniae vaccine is inoculated, an antigen derived from vaccine is exhausted in urine for a few days and be careful because positive test result may be given.
[Test procedure]
● Details of test plate
 ●Test procedure
●Test procedure
1) Preparation of reagent
No prior preparation required.
2) Test procedure
① Take out test plate from aluminum foil pouch.
Remove desiccant sheet included in aluminum foil pouch.
② Absorb urine so that urine can reach between two graduation lines near tip of dropper included in kit. (About 100μL can be collected.) When taking dropper out from urine, get dropper upright and take it out.
③ Add all absorbed urine to specimen area of test plate vertically.
※Add urine of 100μL to test plate in case of using micropipette.
④ Leave to react at 15 ~30℃.
Visually interpret test result by lines appearing in test line area and in control line area after 5 ~10 minutes.
[Interpretation]
Interpret by existence of red-purple lines appearing in test line area and control line area.
《Positive》
Both test line and control line appear.
Positive
《Negative》
Only control line appears.
Negative
《Retest》
If both test lines and control line do not appear or no control line appear, operational mistakes such as the shortage of sample volume etc. are thought about. Recheck test procedure and retest with new test plate. If the same result comes out in the retest again, confirm it with other method.
Retest
Retest
● Interpretational note
1) In case test line and control line appear at 5 ~ 10 minutes after adding sample, it can be interpreted as positive. Negative should be interpreted at 10 minutes after adding sample. Streak line might appear before 10 minutes temporarily. Do not interpret the temporal streak line as appearance of test line. After 10 minutes, colloidal gold can appear like line due to drying of test plate with time. Therefore interpret test results at 10 minutes.
2) This product is used as an aid in the diagnosis for infection of streptococcus pneumoniae. In case that streptococcus pneumoniae antigens in specimen are below the detection limit of the test, test result could be interpreted as negative,
even though patients are infected by streptococcus pneumoniae. Moreover, special factors in specimen could cause non-specific reaction and negative specimen could be interpreted as positive. The definitive diagnosis should be made comprehensively in conjunction with the assessment of clinical progress and other test result.
3) Streptococcus mitis has a common antigen with streptococcus pneumoniae and it is supposed that positive result is given when there is much quantity of the antigen.
4) When streptococcus pneumoniae habitually resides to the nasopharynx in infant, a streptococcus pneumoniae antigen is exhausted in urine and be careful because positive result may be given.
5) It is usually said that quantity of urinary streptococcus pneumoniae antigen reaches detectable sensitivity or more after 3 days after a pneumoniae symptom appeared, but it is different by a symptom. In addition, an antigen may be exhausted in urine from a few days to several weeks after infection.
[Performance Characteristics]
1) Performance
① Sensitivity
・When in-house positive control Note 1) was tested, a positive result was shown.
② Accuracy
・When in-house positive control was tested, a positive result was shown.
・When in-house negative control Note 2) was tested, negative result was shown.
③ Reproducibility
・When in-house positive controls were tested three time simultaneously, positive results were shown in all cases.
・When in-house negative controls were tested three times simultaneously, negative results were shown in all cases.
Note 1) Streptococcus pneumoniae antigen control solution, diluted by artificial urine to be equivalent to 1.0×10 3 CFU/ml of reference material.
Note 2) Aqueous solution including urea and sodium chloride
④ Detection Limit
2.5×10 2 CFU/mL
⑤ Serotype and reactivity
It is confirmed that this product reacts with streptococcus pneumoniae serotype 1, 3, 4, 6B, 9N, 9V, 10A, 11A/E, 12F, 14, 15B, 18C, 19A, 19F, 20, 22F, 23F, 33F.
2) Correlation
Comparison with existing approval product
(immmunochromatographic assay)
Quick Chaser Streptococcus Pneumoniae
Other product
Positive agreement rate : 100%(62/62)
Negative agreement rate :92.7%(51/55)
Total agreement rate :96.6%(113/117)
3) Calibration reference material (Standard material)
Streptococcus pneumoniae (ATCC49619)
[Precaution for use or handling]
1) Precaution for handling (hazard prevention)
①Other infectious materials could be included besides streptococcus pneumoniae in specimen. Be careful of handling specimen as potentially infectious materials.
②Be careful not to touch specimen directly to skin or not to get into eyes in wearing glasses, disposable gloves or mask at the time of use.
③If specimen is got into eyes or mouth, flush with a plenty of water as emergency treatment and see a doctor if necessary.
④Raw material of membrane which is used for test plate, is nitrocellulose. Do not perform test near fire because nitrocellulose is extremely flammable material.
2) Precaution for use
①Avoid freezing of reagent and store it in accordance with description of instruction for use. Do not use frozen reagents because they could show false results by change of quality.
②Do not use this product beyond expiration date.
③Use the test plate immediately after opening aluminum foil pouch. If test plate is left for a long time, it could not react by exposure to moisture.
④Do not touch sample area, test line area and control line area by hands directly.
⑤Do not perform test in the place such as under air conditioner where the dry wind directly blows the surface of the test plate, to prevent uneven migration.
⑥Do not use the reagent and the accessories etc. of this product except the purpose of this testing.
⑦Test plate and dropper are intended for single use only.
3) Precaution for waste disposal
①Treat liquid waste and used utensils by any one of following methods because specimen could contain other infectious material besides streptococcus pneumoniae.
a) Immerse in sodium hypochlorite solution (effective chlorine concentration of 1,000 ppm) for 1 hour or more.
b) Immerse in 2 % glutaraldehyde solution for 1 hour or more.
c) Autoclave at 121℃ for 20 minutes or more.
②Regarding disposal of used reagent and utensils, dispose of them as medical waste, industrial waste or infectious waste in accordance with Local Regulation and Law of waste disposal.
[Storage・Expiry]
・Storage: Room temperature (1~30℃)
・Expiry: 12 months (As indicated on package)














